
Welcome to CMPV !!! Your Trusted Partner in Ensuring Drug Safety and Regulatory Compliance in India !!
At ClovisMed, we are dedicated to providing comprehensive pharmacovigilance solutions tailored to meet the unique needs of pharmaceutical companies, biotech firms, and healthcare organizations in India. With a team of experienced pharmacovigilance experts and regulatory professionals, we offer a range of services to support your drug safety and compliance requirements.
Unique Pharmacovigilance Solutions by CMPV Consultancy LLP
In an increasingly complex global regulatory landscape, pharmacovigilance is no longer just a compliance function—it is a strategic business enabler. CMPV Consultancy LLP delivers specialized, high-value pharmacovigilance solutionsdesigned to protect patient safety, minimize regulatory risk, and strengthen business outcomes.
What Makes CMPV Consultancy LLP Unique?
We combine regulatory excellence, operational precision, and practical implementation, offering solutions that go beyond standard PV services.
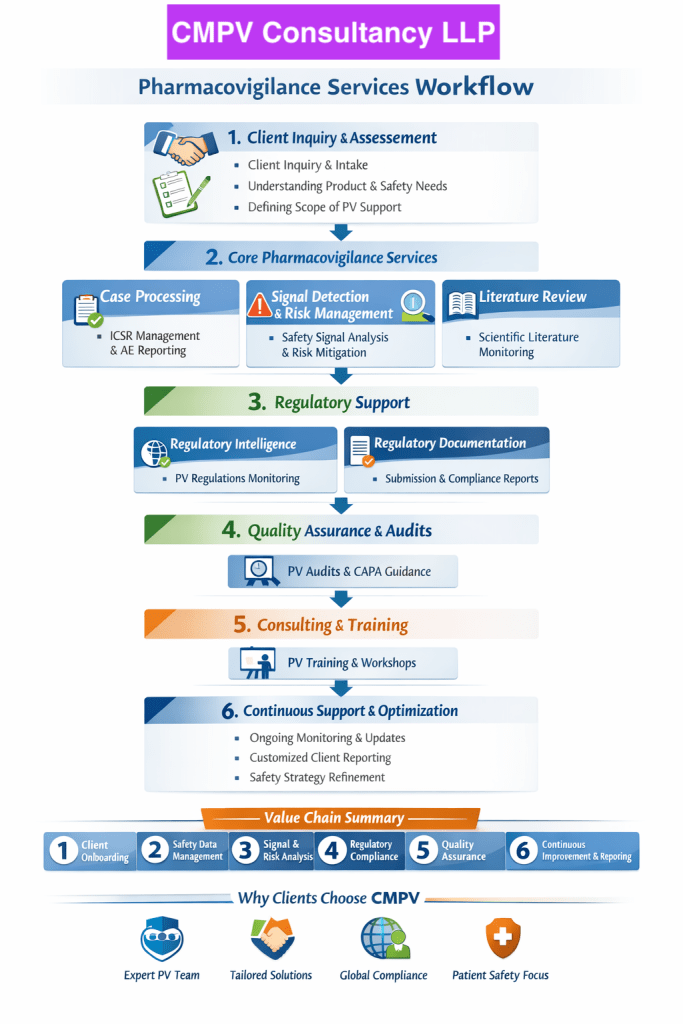
Our Unique Pharmacovigilance Service Portfolio
🔹 Risk-Based Pharmacovigilance Audits
Unlike checklist-driven audits, CMPV conducts risk-based, decision-focused audits aligned with:
- ICH, EU-GVP, US FDA, CDSCO & global regulations
- Business objectives such as M&A, licensing, and vendor selection
Outcome: Clear risk visibility, prioritized CAPAs, and inspection-ready systems.
🔹 Pharmacovigilance Due Diligence for M&A and Licensing
We provide independent PV due diligence audits for:
- Product acquisitions & divestments
- In-licensing / out-licensing deals
- CRO and PV vendor onboarding
Outcome: Early identification of PV liabilities, compliance gaps, and integration risks—before commercial decisions are made.
🔹 End-to-End Pharmacovigilance System Setup
CMPV builds customized, scalable PV systems from the ground up for:
- Start-ups & emerging pharma companies
- Marketing authorization holders (MAHs)
- Companies entering new markets
Includes: SOPs, QMS, safety database readiness, regulatory reporting workflows, and staff training.
🔹 Remote Pharmacovigilance Audits & Inspections
Our secure remote audit model ensures:
- Minimal business disruption
- Faster audit execution
- Global audit coverage
Outcome: Cost-effective, inspection-ready assessments without geographical limitations.
🔹 Source Data Verification (SDV) for Safety Data
CMPV offers specialized SDV services for pharmacovigilance, ensuring:
- Data integrity across safety databases
- Accurate case processing & reporting
- Traceability from source to submission
Outcome: Reliable safety data and reduced regulatory findings.
🔹 Business Continuity Planning (BCP) for PV Operations
We design audit-ready BCP frameworks aligned with:
- ISO 22301
- CDSCO & global inspection expectations
Outcome: Uninterrupted PV operations during crises, audits, or system failures.
🔹 Safety & Complaint Handling Integration
CMPV bridges pharmacovigilance and product complaint handling, ensuring:
- Seamless safety escalation
- Regulatory-compliant reporting
- Improved cross-functional coordination
Outcome: Strong post-marketing surveillance and regulatory confidence.
🔹 Practical CAPA & Remediation Support
We don’t just identify gaps—we help fix them.
CMPV provides:
- Realistic, implementable CAPA plans
- Hands-on remediation support
- Audit and inspection response preparation
Outcome: Sustainable compliance, not temporary fixes.
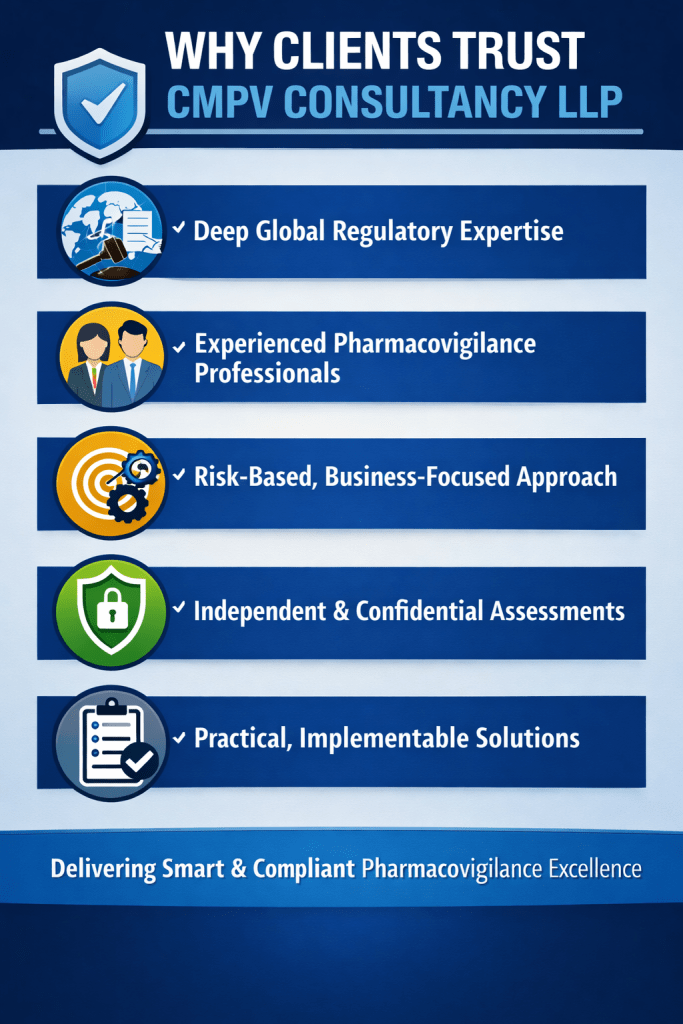
Why Clients Trust CMPV Consultancy LLP
✔ Deep Global Regulatory Expertise
✔ Experienced Pharmacovigilance Professionals
✔ Risk-Based, Business-Focused Approach
✔ Independent & Confidential Assessments
✔ Practical, Implementable Solutions
Who We Serve
- Pharmaceutical & Biotech Companies
- Medical Device Manufacturers
- CROs & PV Service Providers
- Start-ups & Emerging Pharma
- Investors & Business Development Teams
Delivering Value Beyond Compliance
With CMPV Consultancy LLP, you gain:
- Reduced regulatory and commercial risk
- Stronger inspection readiness
- Faster market access
- Improved patient safety oversight
- Long-term pharmacovigilance excellence
Partner with CMPV Consultancy LLP
Your trusted partner for smart, compliant, and future-ready pharmacovigilance solutions.
📩 Contact us today to explore how CMPV’s unique pharmacovigilance services can support your organization’s growth and compliance journey.


Latest updates about CMPV Consultancy LLP
Registration Details



MoU Signed with

PV.app is AI-powered cloud solution designed
to revolutionize the collection and management
of pharmaceutical, vaccine, biologics, veterinary
and other products safety data.
At PV.app the reporting process simpler and to give benefits from Pharmacovigilance for both pharmaceutical companies and individuals.
If you’d like to help and be part of this exciting future, please contact
Kyiv, Tarasivska street, 4-A, office 7info@bioinnova.com.ua
If you’d like to help and be part of this exciting future, please contact











