Have you experienced any side effect, adverse event, or safety issue after using a medical device?
Your report is important for protecting public health and improving the safe use of medical devices in India.
🩺 What can be reported?
- Side effects or adverse events following use of a medical device
- Device malfunction, failure, or quality issues
- Injury, health deterioration, or unexpected outcome
- Any serious or unusual safety concern related to a medical device
Medical devices include:
Implants, stents, catheters, syringes, surgical instruments, diagnostic kits, monitoring devices, implants, and other medical equipment.
📩 How to report?
Send details of the adverse event to:
CMPV Consultancy LLP
📧 Email: cmpvconsultancy@gmail.com
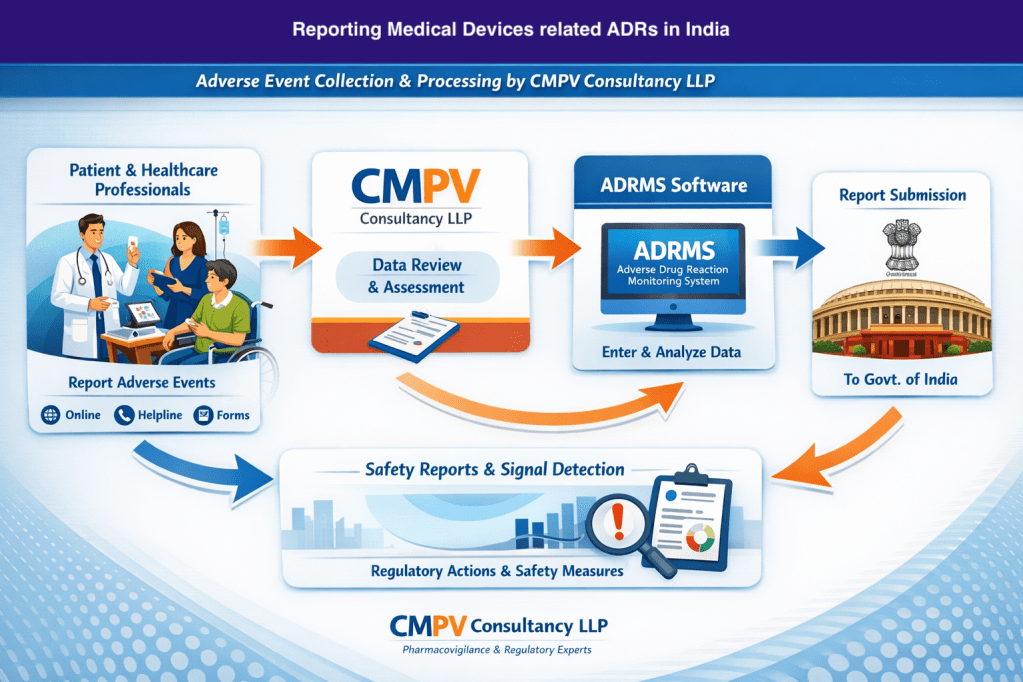
🏛️ What happens to your report?
- Reports are reviewed and assessed by pharmacovigilance professionals
- Valid cases are processed and submitted to the Government of India
- Reporting is done through the ADRMS (Adverse Drug Reaction Monitoring System) as per national guidelines
- Data supports medical device vigilance, signal detection, and regulatory safety actions
🔒 Confidentiality & Eligibility
- Patient confidentiality is strictly maintained
- Reports accepted from patients, caregivers, healthcare professionals, and the general public
📣 Your report can save lives.
📣 Report adverse events today and contribute to medical device safety in India.
—
CMPV Consultancy LLP
Pharmacovigilance & Medical Device Vigilance Experts

Leave a comment