Ensuring Data Accuracy, Integrity & Regulatory Compliance
At CMPV Consultancy LLP, our Source Data Verification (SDV) process is designed to ensure the accuracy, completeness, consistency, and traceability of safety and clinical data across pharmacovigilance and clinical operations. SDV plays a critical role in maintaining data integrity, supporting regulatory submissions, and ensuring patient safety.
Our structured, risk-based SDV approach complies with ICH-GCP (E6 R2/R3), EU GVP, CDSCO, US FDA, and global regulatory inspection requirements.
What is Source Data Verification?
Source Data Verification is the systematic comparison of source documents (e.g., medical records, laboratory reports, case report forms, safety databases) with reported data to confirm that information is:
- Accurate
- Complete
- Consistent
- Timely
- Traceable to original records
CMPV Consultancy LLP – SDV Approach
We follow a risk-based, audit-ready SDV methodology tailored to sponsor, CRO, and marketing authorization holder (MAH) requirements.
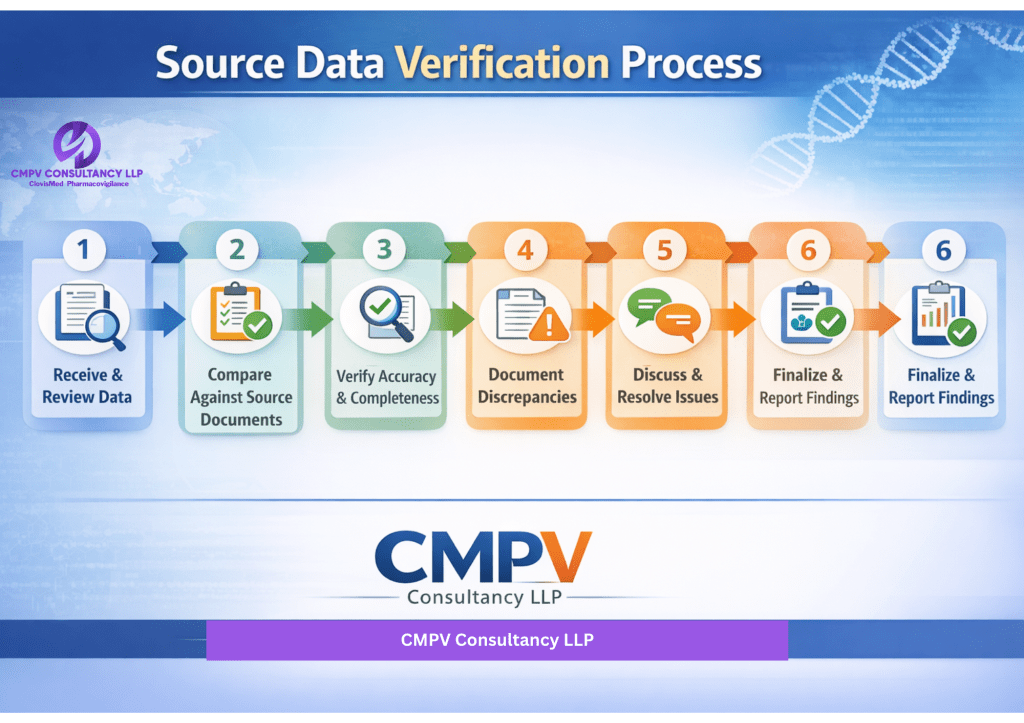
1. SDV Planning & Scope Definition
- Review of protocol, safety management plans, SOPs, and data flow
- Identification of critical data points (SAEs, AESIs, endpoints)
- Determination of SDV intensity (100% or targeted/risk-based)
- Development of SDV plan and checklist
2. Source Document Review
- Verification of source documents including:
- Medical records and case notes
- Laboratory and diagnostic reports
- Informed consent forms
- Adverse event and SAE documentation
- Confirmation of document authenticity, legibility, and completeness
3. Data Comparison & Verification
- Cross-checking source data against:
- Case Report Forms (CRFs/eCRFs)
- Safety databases (Argus, ArisG, etc.)
- Line listings and narratives
- Verification of:
- Event onset and resolution dates
- Seriousness, severity, causality
- Concomitant medications and medical history
4. Query Identification & Resolution
- Identification of discrepancies, missing data, or inconsistencies
- Generation of data clarification queries
- Coordination with site, sponsor, or safety teams for resolution
- Documentation of query closure and justification
5. Documentation & Traceability
- Maintenance of SDV logs and evidence trails
- Verification of audit trail entries
- Confirmation of data version control and change history
- Ensuring ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate)
6. SDV Reporting & Compliance Review
- Preparation of detailed SDV reports including:
- Observations and findings
- Risk classification
- Corrective and preventive action (CAPA) recommendations
- Readiness support for regulatory inspections and audits
Key Benefits of CMPV’s SDV Services
- ✔ Improved data quality and reliability
- ✔ Reduced regulatory and inspection risk
- ✔ Early detection of data gaps and compliance issues
- ✔ Support for pharmacovigilance audits and submissions
- ✔ Enhanced confidence in safety signal evaluation
Applicable Regulatory Standards
- ICH-GCP E6 (R2/R3)
- EU GVP Modules
- US FDA 21 CFR Part 11
- CDSCO & DCGI requirements
- ISO-aligned quality systems
Why Choose CMPV Consultancy LLP?
- Experienced pharmacovigilance and clinical QA professionals
- Risk-based and inspection-ready SDV methodology
- Remote and on-site SDV support
- Customized solutions for sponsors, CROs, and MAHs
- Strong focus on data integrity and patient safety
Contact Us
If you are looking for reliable, compliant, and efficient Source Data Verification services, CMPV Consultancy LLPis your trusted partner.
📧 Email: cmpvconsultancy@gmail.com
🌐 Website: http://www.cmpv.in
Contact us today to strengthen your data integrity and regulatory compliance.

Leave a comment