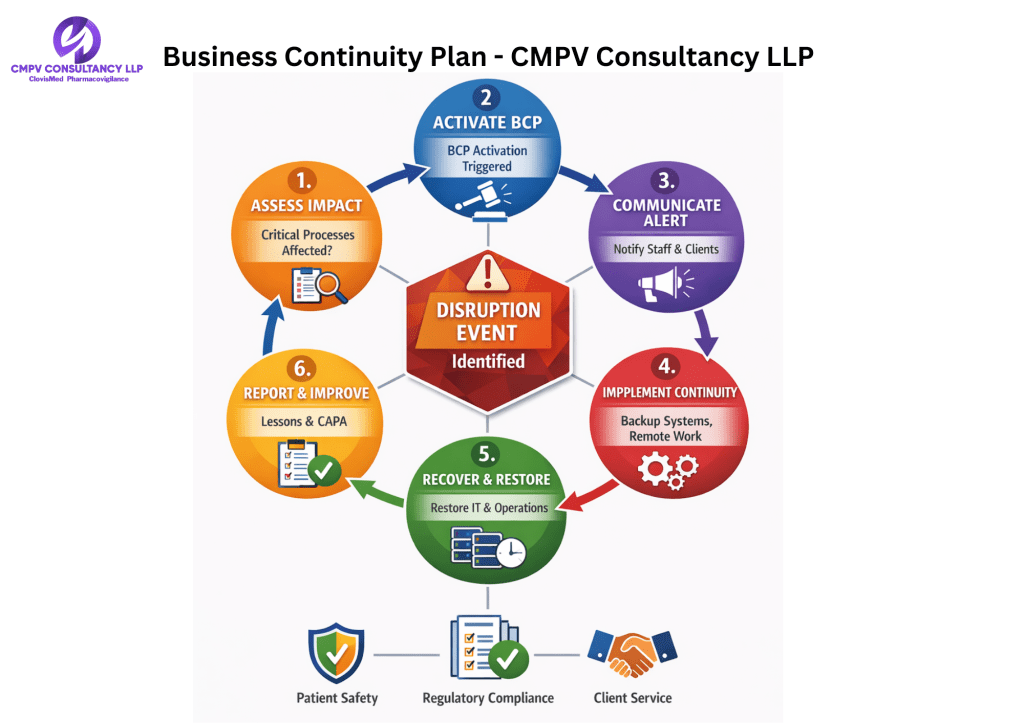
Our Commitment to Continuity & Compliance
CMPV Consultancy LLP is committed to ensuring uninterrupted delivery of pharmacovigilance, regulatory, and safety services under all circumstances. Our Business Continuity Plan (BCP) is designed to safeguard client operations, patient safety, data integrity, and regulatory compliance during any disruptive event.
This plan aligns with ICH, WHO, ISO 22301 principles, and applicable global pharmacovigilance regulatory expectations.
Purpose of the Business Continuity Plan
The purpose of CMPV’s Business Continuity Plan is to:
- Ensure continuity of critical pharmacovigilance activities
- Minimize disruption to client deliverables
- Protect patient safety and regulatory timelines
- Maintain data security and confidentiality
- Enable rapid recovery from operational disruptions
Scope of the BCP
This plan applies to all CMPV Consultancy LLP operations, including:
- Individual Case Safety Report (ICSR) processing
- Signal detection and risk management
- Aggregate report preparation (PSUR, PBRER, DSUR)
- Regulatory submissions and safety reporting
- Medical review and quality oversight
- Pharmacovigilance audits and inspections
- Training, documentation, and compliance support
The BCP covers both onshore and remote operations.
Risk Scenarios Covered
CMPV’s BCP addresses potential risks including but not limited to:
- Natural disasters (floods, earthquakes, pandemics)
- IT system failures or cyber incidents
- Power outages or infrastructure disruptions
- Workforce unavailability
- Regulatory or travel restrictions
- Third-party service interruptions
Business Impact Analysis (BIA)
A structured Business Impact Analysis is conducted to:
- Identify critical business processes
- Define Recovery Time Objectives (RTO)
- Define Recovery Point Objectives (RPO)
- Prioritize pharmacovigilance activities impacting patient safety and compliance
Critical processes are classified and protected with predefined recovery strategies.
Continuity & Recovery Strategies
CMPV Consultancy LLP ensures resilience through:
- Remote-ready workforce model
- Secure cloud-based pharmacovigilance systems
- Redundant IT infrastructure and data backups
- Cross-trained pharmacovigilance professionals
- Alternate communication channels
- Controlled access to validated safety databases
IT & Data Protection
- Regular automated data backups
- Secure VPN and access-controlled environments
- Data integrity checks and validation controls
- Compliance with GDPR, local data protection laws, and confidentiality agreements
Crisis Management & Governance
A dedicated BCP Response Team is responsible for:
- Incident assessment and escalation
- Communication with clients and stakeholders
- Activation of continuity measures
- Monitoring recovery progress
- Documentation and post-incident review
Roles and responsibilities are clearly defined and periodically reviewed.
Communication During Disruption
CMPV ensures transparent and timely communication through:
- Predefined escalation and contact matrices
- Client notification protocols
- Regular status updates during incidents
- Single-point contact for continuity coordination
Training, Testing & Review
- Periodic BCP awareness training for employees
- Mock drills and continuity testing
- Continuous improvement based on lessons learned
- Annual review or revision upon significant operational changes
Regulatory & Client Assurance
CMPV’s Business Continuity Plan is:
- Available for client audits and regulatory inspections
- Integrated into the Quality Management System
- Aligned with global pharmacovigilance expectations
- Designed to protect patient safety above all
Conclusion
CMPV Consultancy LLP is fully prepared to maintain uninterrupted pharmacovigilance and regulatory services, even during unforeseen disruptions. Our robust Business Continuity Plan ensures resilience, reliability, and regulatory confidence for our global clients.
