Business Model
CMPV Consultancy LLP (also presented as ClovisMed Pharmacovigilance Consultancy) is an India-based professional services firm specializing in pharmacovigilance — the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems.
Founded in March 2024 and registered as an LLP with the Registrar of Companies in Cuttack, Odisha, India, the firm operates with a focus on supporting pharmaceutical companies, biotech organizations, healthcare institutions, and related stakeholders in ensuring drug safety and regulatory compliance.
🧩 1. Value Proposition
CMPV Consultancy’s core business proposition centers on delivering expert pharmacovigilance services that help clients:
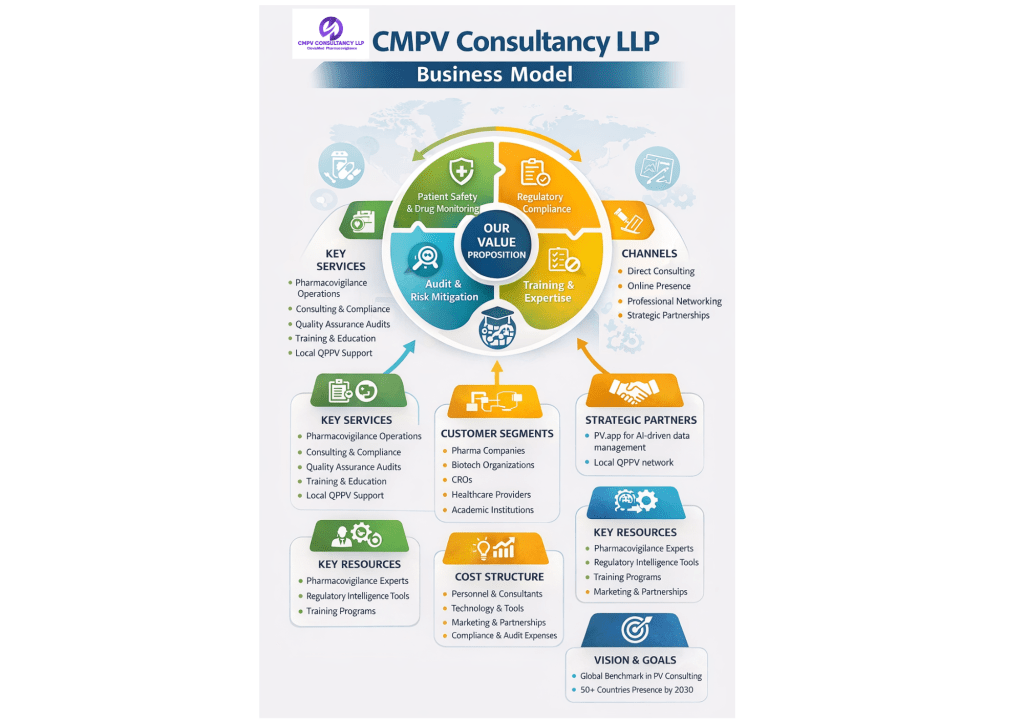
- Maintain patient safety by ensuring effective monitoring and reporting of adverse drug events.
- Ensure regulatory compliance with both local (e.g., CDSCO-India) and global health authority standards.
- Improve internal safety systems through audits, risk mitigation strategies, and documentation support.
- Build organizational capability via training, intelligence, and qualified personnel support.
The company emphasizes expertise, customized solutions, confidentiality, and client-centric service delivery as core differentiators.
🛠️ 2. Key Services (Revenue Streams)
CMPV generates revenue by offering a suite of specialized service offerings in pharmacovigilance, including:
🔹 Pharmacovigilance Operational Services
- Individual Case Safety Report (ICSR) management
- Adverse Event Reporting & processing
- Signal detection and risk management
These services help clients manage and report safety data accurately and timely.
🔹 Consulting & Compliance Services
- Regulatory compliance support
- Regulatory intelligence & strategy
- Benefit-risk assessments
These ensure alignment with evolving regulatory frameworks and minimize risk of non-compliance.
🔹 Quality Assurance & Audit Services
- Internal pharmacovigilance audits
- Risk-based audits
- Remote audit support
- Corrective & Preventive Action (CAPA) guidance
CMPV assesses and strengthens clients’ internal pharmacovigilance system quality.
🔹 Training & Education
- Pharmacovigilance training programs tailored for client teams
This builds internal capability in drug safety and compliance.
🔹 Local QPPV (Qualified Person for Pharmacovigilance) Support
- Provision of QPPV expertise
- 24/7 compliance stewardship
- Reporting & documentation services
This is particularly suited for companies requiring in-region regulatory oversight.
👥 3. Customer Segments
CMPV serves a range of clients, including:
- Pharmaceutical companies (large, mid-size, and emerging startups)
- Biotech & medical product manufacturers
- Healthcare organizations and hospitals
- Contract Research Organizations (CROs)
- Academic institutions needing pharmacovigilance support or training
🔑 4. Channels
CMPV engages clients and delivers services through:
- Direct consulting engagements
- Collaborative partnerships and MOUs (e.g., with PV.app for AI-driven data management tools)
- Online presence via official site and digital outreach
- Professional networks in the pharmaceutical and regulatory domain
- Training workshops and customized engagements
🤝 5. Strategic Partnerships & Innovation
- International collaboration with PV.app — an AI-powered platform to improve data entry and reporting workflows, which enhances the firm’s technology offering.
This partnership expands CMPV’s service delivery model into technology-enabled pharmacovigilance solutions — enabling faster, compliant data submissions and improved quality control.
🧠 6. Key Resources
To operate its consultancy business, CMPV leverages:
- Expert pharmacovigilance professionals and regulatory specialists
- Training content and frameworks for internal and client learning
- Intellectual property in audit methods, reporting processes, and compliance methodologies
- Technology partnerships (e.g., PV.app platform)
🏭 7. Cost Structure
Major ongoing costs likely include:
- Salaries and professional consultant fees
- Regulatory and compliance research tools
- Audit and reporting infrastructure
- Marketing and business development expenses
- Training development and delivery costs
- Technology platform integration and support
📊 8. Competitive Advantage
CMPV distinguishes itself through:
- Niche specialization in pharmacovigilance — not general consulting.
- Deep regulatory expertise tailored for drug safety and global compliance.
- Comprehensive service offerings from operational to strategic consulting.
- Technology integration (AI-assisted reporting) through partnerships.
🎯 9. Vision & Long-Term Goals
CMPV aims to:
- Become a global benchmark in pharmacovigilance consulting
- Expand its services across 50+ countries by 2030
- Enhance drug safety culture and systemic compliance in the healthcare sector
Their mission emphasizes patient safety and professional development in the field.
