Preserving Data Integrity, Compliance, and Inspection Readiness
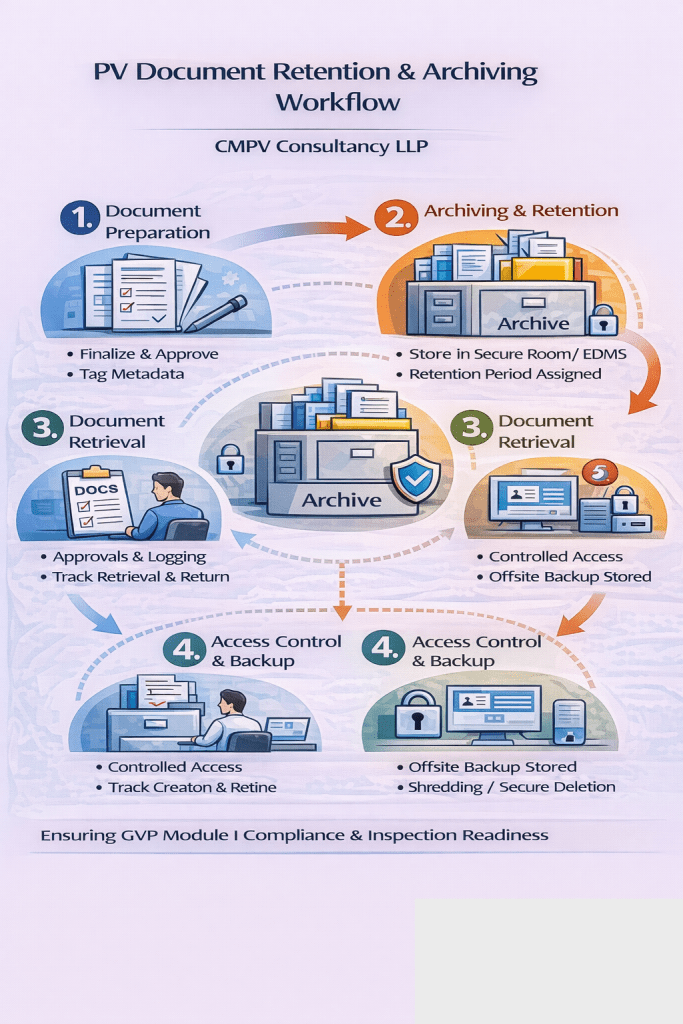
At CMPV Consultancy LLP, we support pharmaceutical and biotechnology companies in establishing and maintaining compliant document retention and archiving systems for pharmacovigilance (PV) documents. Proper retention and secure archiving of PV records are essential to demonstrate regulatory compliance, ensure data integrity, and support ongoing patient safety activities.
Importance of Document Retention & Archiving in Pharmacovigilance
Pharmacovigilance documentation must be retained and readily retrievable for regulatory inspections, audits, and safety evaluations. Regulatory authorities expect PV documents to be:
- Complete and accurate
- Secure and protected from loss or unauthorized access
- Easily retrievable throughout the retention period
- Archived in accordance with global regulations
Failure to comply may result in inspection findings, regulatory actions, or data integrity concerns.
Regulatory Expectations
CMPV Consultancy LLP aligns PV document retention and archiving practices with:
- EU-GVP Modules I & VI
- ICH E2 guidelines
- US FDA 21 CFR
- MHRA requirements
- CDSCO and local regulatory guidelines
Retention periods may vary based on region, product type, and document category.
Pharmacovigilance Documents Requiring Retention
🔹 Core PV System Documents
- Pharmacovigilance System Master File (PSMF)
- Quality manuals and SOPs
- Training records
🔹 Safety Case Documentation
- Individual Case Safety Reports (ICSRs)
- Source documents and follow-up records
- Medical assessments and narratives
🔹 Aggregate and Risk Management Documents
- PSUR / PBRER / DSUR
- Risk Management Plans (RMPs)
- Signal management documentation
🔹 Compliance and Oversight Records
- Audit and inspection reports
- CAPA documentation
- Regulatory correspondence
Retention Periods (General Guidance)
- ICSRs and source data: Minimum of 10 years after product withdrawal or as per local regulation
- PSMF and quality documents: For the lifetime of the system and defined post-closure period
- Audit and CAPA records: As per regulatory and internal quality requirements
CMPV Consultancy LLP assists clients in defining region-specific and product-specific retention timelines.
Archiving Best Practices in Pharmacovigilance
🔹 Secure Archiving
- Controlled access and authorization
- Protection from loss, damage, or unauthorized modification
- Disaster recovery and backup systems
🔹 Electronic and Paper Archives
- Validation of electronic archiving systems
- Clear indexing and document traceability
- Proper labeling and storage conditions for paper records
🔹 Retrieval and Inspection Readiness
- Rapid retrieval during audits and inspections
- Clear audit trails and version control
- Periodic archive review and reconciliation
Our Document Retention & Archiving Services
CMPV Consultancy LLP provides comprehensive support including:
✔ Development of PV document retention and archiving SOPs
✔ Retention schedule definition and implementation
✔ Electronic archive system compliance support
✔ Archival audits and gap assessments
✔ Inspection readiness and regulatory support
✔ Secure transition from active to archived records
✔ Data integrity and GDP compliance checks
Why Choose CMPV Consultancy LLP?
- Expertise in global pharmacovigilance regulations
- Strong focus on data integrity and inspection readiness
- Practical, scalable solutions for organizations of all sizes
- End-to-end support from SOP development to archive audits
- Commitment to quality and patient safety
Our Commitment
At CMPV Consultancy LLP, we ensure that pharmacovigilance documents remain secure, compliant, traceable, and accessible throughout their lifecycle. Our structured approach to document retention and archiving supports regulatory compliance while strengthening overall PV system quality.
Contact Us
For expert support on Document Retention & Archiving of Pharmacovigilance Documents, contact CMPV Consultancy LLP.
