Good Documentation Practice (GDP) in Pharmacovigilance
Good Documentation Practice (GDP)
Ensuring Data Integrity, Compliance, and Patient Safety
At CMPV Consultancy LLP, we emphasize the critical role of Good Documentation Practice (GDP) in maintaining a robust and compliant pharmacovigilance (PV) system. Accurate, complete, and reliable documentation is the foundation of regulatory compliance, inspection readiness, and effective patient safety management.
What is Good Documentation Practice (GDP)?
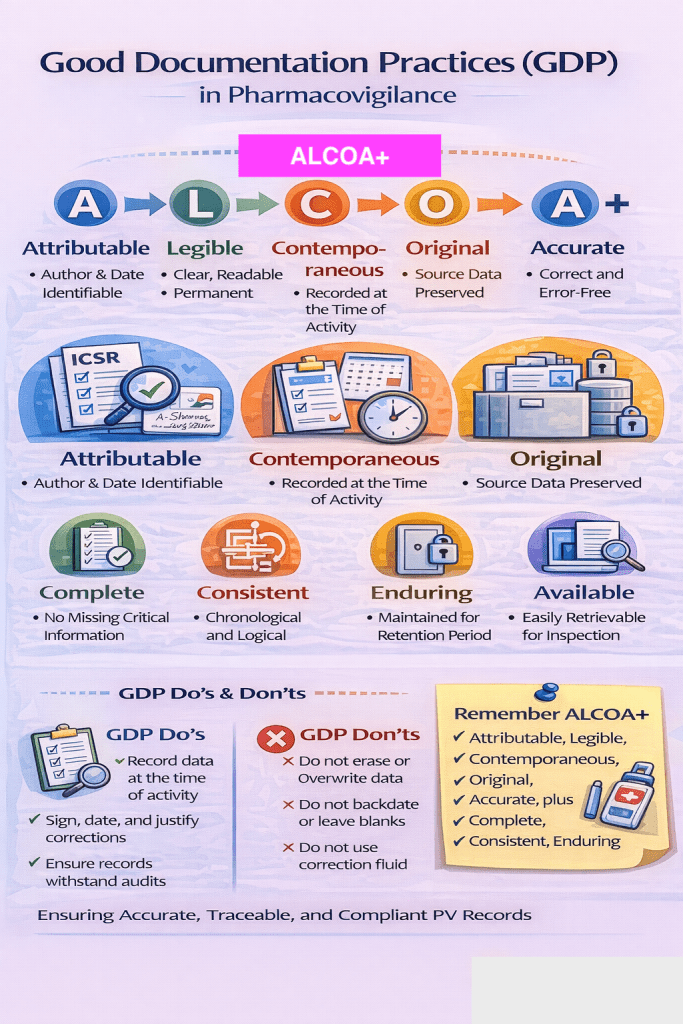
Good Documentation Practice refers to the principles and standards that ensure all pharmacovigilance records are:
- Accurate
- Complete
- Legible
- Traceable
- Contemporaneous
- Consistent
GDP ensures that pharmacovigilance data can be trusted by regulatory authorities and used effectively for safety evaluation and decision-making.
Importance of GDP in Pharmacovigilance
- Ensures compliance with ICH-GCP, ICH E2 guidelines, EU-GVP, US FDA, MHRA, and CDSCO requirements
- Supports accurate case processing and regulatory submissions
- Enhances data integrity and audit readiness
- Enables transparent safety signal detection and risk management
- Protects patient safety and organizational credibility
Key Principles of Good Documentation Practice
CMPV Consultancy LLP follows internationally accepted GDP principles, including:
🔹 ALCOA+ Principles
- Attributable – Clear identification of who recorded the data
- Legible – Readable and understandable documentation
- Contemporaneous – Recorded at the time the activity is performed
- Original – First capture or certified true copy
- Accurate – Correct and error-free
Plus: Complete, Consistent, Enduring, and Available
GDP Requirements in Pharmacovigilance Activities
🔹 Individual Case Safety Reports (ICSRs)
- Accurate source documentation
- Clear data entry and medical assessment
- Proper version control and audit trails
- Timely documentation of follow-up activities
🔹 Safety Databases and Electronic Records
- Controlled access and user roles
- Audit trails for data entry and modification
- Backup, archiving, and data security procedures
🔹 Standard Operating Procedures (SOPs)
- Controlled SOP creation, review, approval, and revision
- Version control and change history
- Training documentation linked to SOP updates
🔹 Aggregate Reports and Risk Management Documents
- Documented data sources and methodologies
- Consistent and traceable safety evaluations
- Approved and archived final versions
🔹 CAPA and Audit Documentation
- Clear identification of deviations and root causes
- Documented corrective and preventive actions
- Timelines, effectiveness checks, and approvals
Common GDP Errors in Pharmacovigilance
CMPV Consultancy LLP helps organizations avoid frequent GDP deficiencies such as:
- Missing or incomplete documentation
- Back-dated or undocumented corrections
- Uncontrolled document versions
- Lack of signatures or approvals
- Inconsistent data across PV systems
Our GDP Support Services
CMPV Consultancy LLP provides expert support in:
✔ Development and review of GDP-compliant SOPs
✔ Pharmacovigilance documentation audits and gap analysis
✔ GDP training for pharmacovigilance professionals
✔ Inspection and audit readiness support
✔ Remediation of GDP deficiencies
✔ Electronic PV system documentation compliance
Why Choose CMPV Consultancy LLP?
- Experienced pharmacovigilance and quality experts
- Strong understanding of global regulatory expectations
- Practical, inspection-ready documentation approach
- Customized GDP solutions for all PV system sizes
- Commitment to quality, compliance, and patient safety
Our Commitment to Quality
At CMPV Consultancy LLP, we believe that “If it is not documented, it is not done.”
We are dedicated to strengthening pharmacovigilance systems through robust documentation practices that withstand regulatory scrutiny and support effective drug safety management.
Contact Us
For expert guidance on Good Documentation Practice in Pharmacovigilance, contact CMPV Consultancy LLP today.
