Risk Management Plan (RMP) Services
CMPV Consultancy
Ensuring Patient Safety Through Proactive Risk Management
At CMPV Consultancy LLP, we provide comprehensive Risk Management Plan (RMP) services to help pharmaceutical, biotechnology, and medical device companies identify, evaluate, minimize, and monitor risks associated with medicinal products throughout their lifecycle. Our expert-driven approach ensures full compliance with global regulatory requirements while prioritizing patient safety.
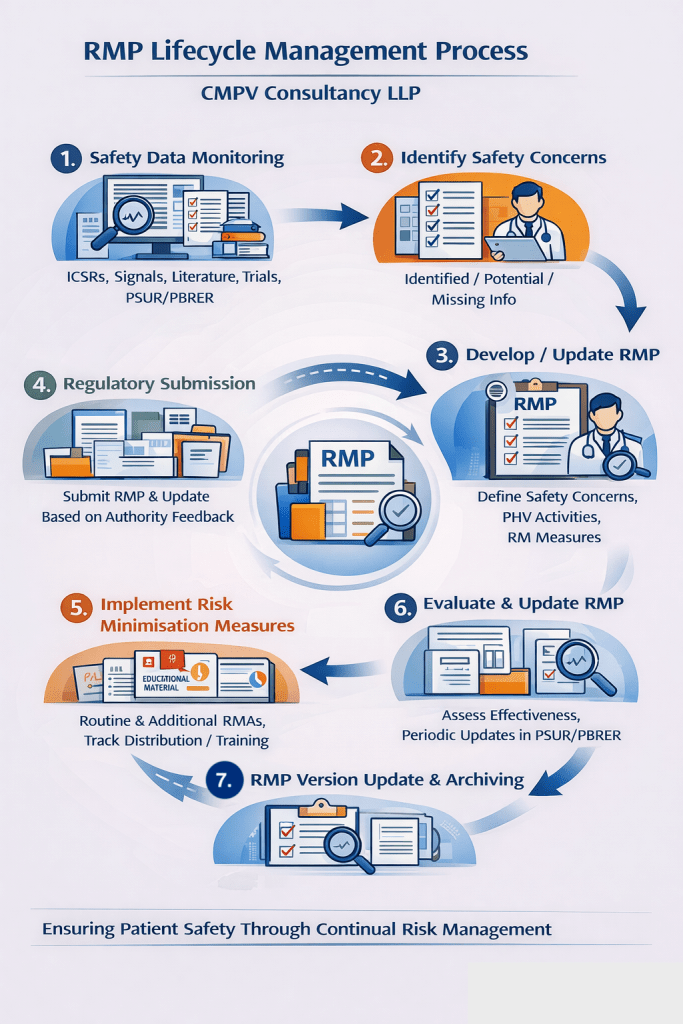
What is a Risk Management Plan (RMP)?
A Risk Management Plan is a structured pharmacovigilance document that outlines:
- Identified and potential risks of a medicinal product
- Missing information related to safety
- Pharmacovigilance activities to monitor safety
- Risk minimization measures to prevent or reduce harm
RMPs are mandatory for regulatory submissions to authorities such as EMA, MHRA, CDSCO, and other global health agencies.
Our RMP Services
🔹 RMP Authoring and Submission
- Preparation of EU-RMP, UK-RMP, and country-specific RMPs
- Alignment with GVP Module V and ICH guidelines
- RMP preparation for new marketing authorization applications, variations, and renewals
🔹 Risk Identification and Evaluation
- Identification of important identified risks, important potential risks, and missing information
- Benefit–risk evaluation based on clinical, post-marketing, and real-world data
🔹 Pharmacovigilance Plan Development
- Design of routine and additional pharmacovigilance activities
- Signal detection strategies and safety monitoring plans
- Integration with PSUR/PBRER and signal management activities
🔹 Risk Minimization Measures (RMMs)
- Development of routine risk minimization strategies
- Design and evaluation of additional RMMs such as:
- Educational materials
- Prescriber guides
- Patient alert cards
- Effectiveness evaluation of risk minimization measures
🔹 RMP Maintenance and Updates
- Periodic review and update of RMPs based on:
- New safety signals
- Regulatory requests
- Post-authorization safety data
- Lifecycle management support
🔹 Regulatory Intelligence & Compliance
- Support during health authority queries and inspections
- RMP alignment with regulatory feedback and evolving guidelines
- Gap analysis and remediation of existing RMPs
Why Choose Us?
✔ Experienced pharmacovigilance and regulatory professionals
✔ Strong understanding of global RMP regulations
✔ High-quality, audit-ready documentation
✔ Customized, product-specific risk management strategies
✔ Timely delivery and dedicated client support
Who Can Benefit from Our RMP Services?
- Pharmaceutical and biotechnology companies
- Generic and biosimilar manufacturers
- Marketing Authorization Holders (MAHs)
- Start-ups and virtual pharma companies
- Clinical research organizations (CROs)
Our Commitment
We are committed to supporting our clients in maintaining a robust risk management framework that ensures regulatory compliance, protects patients, and strengthens product safety throughout the lifecycle.
Contact Us
For expert support on Risk Management Plans in Pharmacovigilance, contact CMPV Consultancy LLP today.
📧 Email: chinmaya.mahapatra@cmpv.in
📞 Phone: +919337504500
