– Partner with CMPV Consultancy LLP
Is your pharmaceutical company prepared to meet global pharmacovigilance (PV) compliance requirements?
Many pharmaceutical and biotechnology companies face challenges in setting up a compliant and sustainable pharmacovigilance system—especially during early market entry, product expansion, or regulatory inspections.
CMPV Consultancy LLP offers end-to-end Pharmacovigilance setup and operational support, tailored to your organization’s needs.
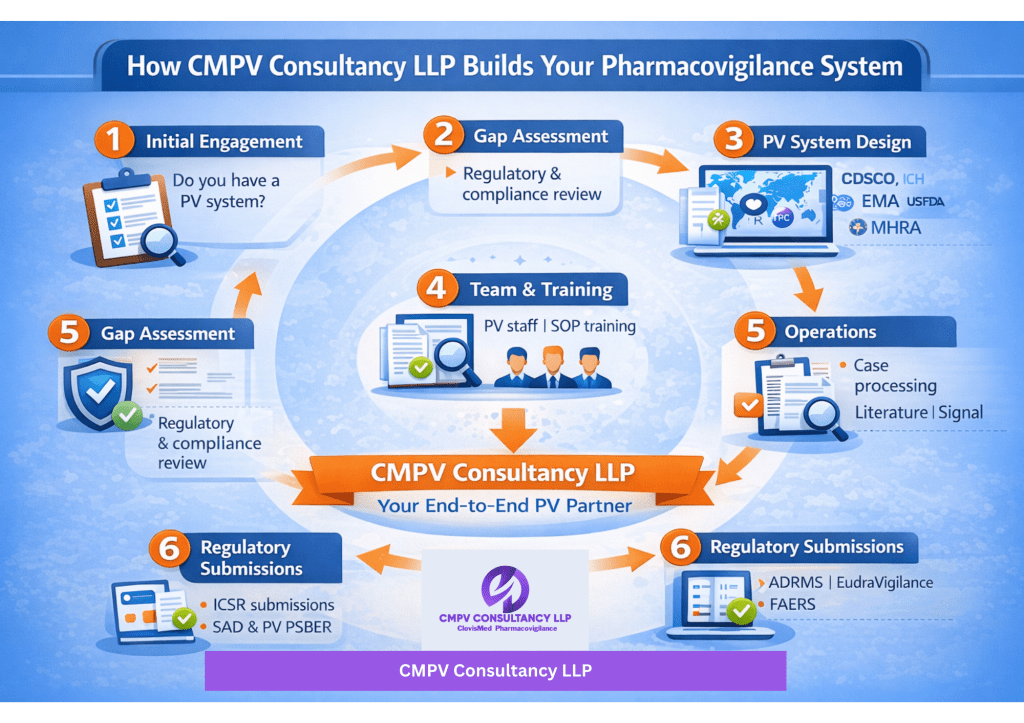
How CMPV Consultancy LLP Works
🔹 Step 1: Initial Engagement & Assessment
We begin with a structured discussion to understand:
- Whether your organization has an existing PV system
- Current regulatory obligations (India & Global)
- Product portfolio and market presence
🔹 Step 2: Gap Analysis & Readiness Check
If a PV system is not in place, CMPV evaluates:
- Regulatory gaps
- Resource requirements
- Compliance risks
- Readiness for audits and inspections
If You Wish to Establish a Pharmacovigilance Setup – CMPV Supports You End-to-End
✅ PV System Design & Implementation
- Complete Pharmacovigilance system setup from scratch
- Alignment with CDSCO, EMA, MHRA, US FDA, and ICH guidelines
✅ PV Team Creation & Staffing Support
- Define PV organizational structure
- Role-based responsibility matrix (QPPV, safety associates, reviewers)
- Support in hiring and onboarding PV professionals
✅ Training & Competency Development
- Initial and ongoing PV training programs
- SOP-based training and regulatory compliance sessions
- Audit and inspection readiness training
✅ SOPs, Policies & Documentation
- Development of PV SOPs, Work Instructions, and Policies
- Safety Management File (SMF) creation
- PV agreements (SDEAs, QPPV agreements, CRO oversight SOPs)
✅ Operational PV Support
- Case intake, processing, and medical review
- Literature screening
- Signal management and risk management support
✅ Regulatory Submissions & Compliance
- ICSR submissions (ADRMS, EudraVigilance, FAERS, etc.)
- Aggregate reports (PBRER, PSUR, DSUR)
- Regulatory intelligence and submission tracking
Why CMPV Consultancy LLP?
✔ Expertise-led PV consulting
✔ Scalable solutions for startups to large pharma
✔ India-focused and global regulatory knowledge
✔ Audit-ready, inspection-compliant systems
✔ Cost-effective and sustainable PV operations
Start Your Pharmacovigilance Journey with Confidence
Whether you are starting from zero or strengthening an existing PV system, CMPV Consultancy LLP becomes your trusted pharmacovigilance partner—from setup to submission and beyond.
📩 Contact CMPV Consultancy LLP today
Let us help you build a compliant, efficient, and future-ready pharmacovigilance system.

Leave a comment