CMPV Consultancy LLP provides independent, risk-based Remote Pharmacovigilance Audit services to support pharmaceutical companies, biotechnology firms, CROs, and marketing authorization holders (MAHs) in ensuring continuous compliance with global pharmacovigilance regulatory requirements.
Our remote audits are designed to deliver the same level of rigor, depth, and regulatory credibility as on-site audits, while offering flexibility, cost efficiency, and rapid execution.
Why Remote Pharmacovigilance Audits?
Remote audits enable organizations to:
- Maintain regulatory oversight during travel or operational constraints
- Ensure continuous PV compliance across global operations
- Identify compliance gaps early and mitigate inspection risks
- Fulfil sponsor oversight obligations for outsourced PV activities
CMPV’s remote audit methodology follows risk-based audit principles and aligns with ICH E2E, GVP Modules (I–XVI), CDSCO PV guidelines, and ISO 19011 auditing principles.
Scope of Remote Pharmacovigilance Audit Services
Our remote PV audits may include, but are not limited to:
- Pharmacovigilance System Audit
- PV system structure and governance
- QPPV / Local Safety Officer oversight
- Safety management and escalation pathways
- Vendor / CRO / Service Provider Audits
- Safety data processing vendors
- Call centers, literature search vendors
- Regulatory submission partners
- SOP & Documentation Review
- SOP compliance and lifecycle management
- Work instructions, templates, logs, and records
- Training documentation and competency assessments
- ICSR Management & Case Processing Audit
- Case intake, triage, coding, assessment
- Timelines compliance and quality checks
- Medical review and follow-up management
- Regulatory Reporting & Submissions
- Expedited reporting (SUSARs, SAEs)
- Periodic safety reports (PSUR/PBRER/DSUR)
- Submission compliance to CDSCO, EMA, FDA, and other authorities
- Signal Management & Risk Management
- Signal detection and evaluation processes
- Risk minimization measures
- Benefit–risk assessment documentation
- Business Continuity & Data Integrity
- BCP and disaster recovery readiness
- Data integrity and access controls
- Audit trail and system compliance
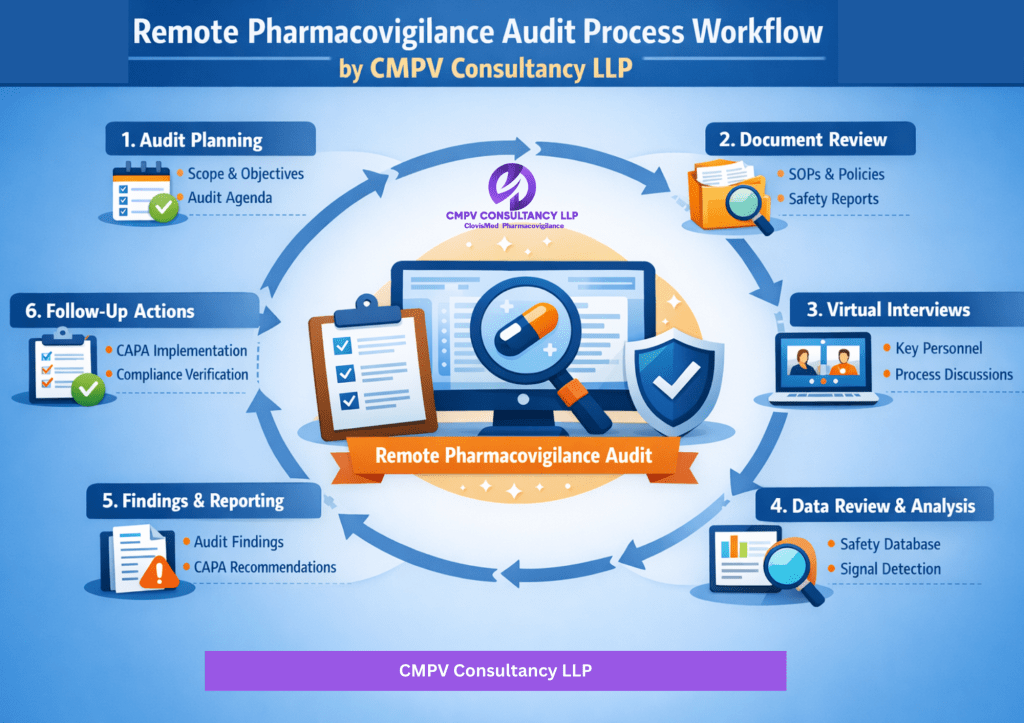
CMPV Remote Audit Approach
Our structured remote audit process includes:
- Pre-Audit Planning & Risk Assessment
- Secure Remote Access & Document Review
- Virtual Interviews with Key PV Personnel
- Sampling-based Case & Process Review
- Gap Analysis & Root Cause Identification
- Audit Report with Risk-Based Findings
- Corrective and Preventive Action (CAPA) Support
All audits are conducted using secure digital platforms with strict confidentiality and data protection controls.
Key Deliverables
- Remote Audit Plan & Agenda
- Audit Checklists aligned to regulatory expectations
- Detailed Audit Report with Critical, Major & Minor Findings
- CAPA recommendations and compliance roadmap
- Management summary for senior leadership
Why Choose CMPV Consultancy LLP?
- Specialized expertise in Pharmacovigilance & Regulatory Compliance
- Experienced auditors with global inspection exposure
- Risk-based, inspection-ready audit methodology
- Independent, confidential, and objective assessments
- Flexible engagement models for startups to global MAHs
Who Can Benefit?
- Pharmaceutical & biotechnology companies
- Marketing Authorization Holders (MAHs)
- CROs & PV service providers
- Virtual / outsourced PV setups
- Companies preparing for regulatory inspections
Contact Us
If you require Remote Pharmacovigilance Audit services, or wish to assess the compliance status of your PV system, CMPV Consultancy LLP is ready to support you.
📩 Contact us today to discuss your audit requirements and receive a customized audit proposal.

Leave a comment