Safety & Complaint Handling
Made Compliant. Reliable. Audit-Ready.
Is your organization fully compliant with safety reporting and product complaint regulations?
At CMPV Consultancy LLP, we provide end-to-end Safety & Complaint Handling services to ensure regulatory compliance, patient safety, and brand protection—without operational burden.
✅ Our Expertise Includes:
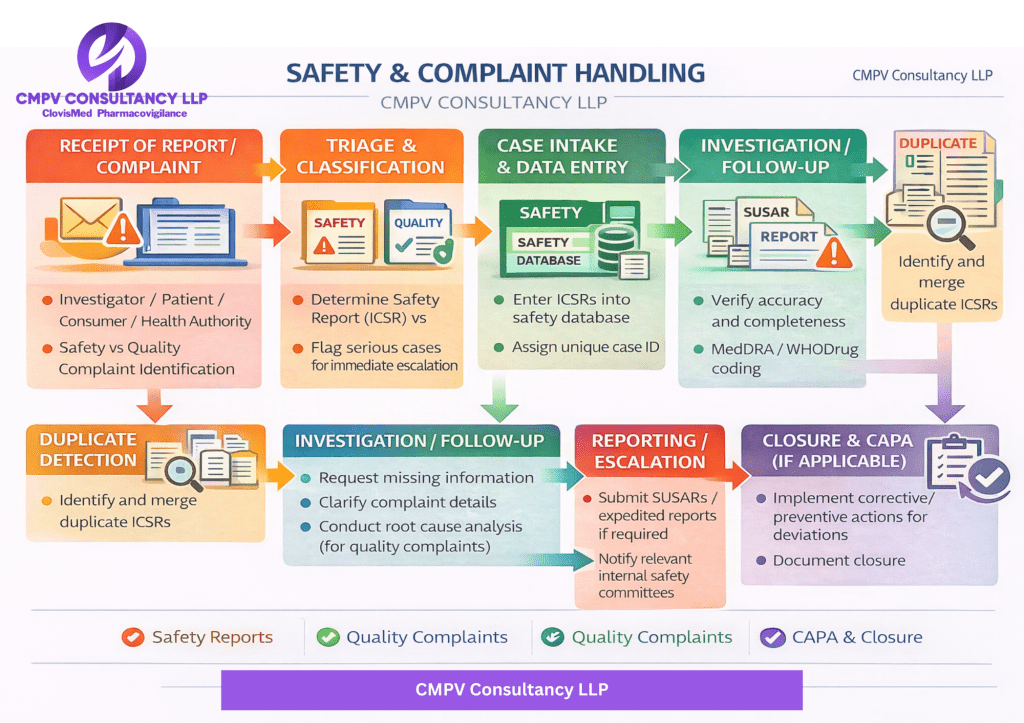
• Adverse Event & Product Complaint intake
• Case triage, medical review & classification
• Timely regulatory reporting (ICSRs)
• Trend analysis & signal detection support
• SOP development & workflow implementation
• Complaint investigations & CAPA management
• Audit-ready documentation and compliance checks
🌍 Why Choose CMPV Consultancy LLP?
✔ Global pharmacovigilance expertise
✔ Aligned with CDSCO, US FDA, EMA & WHO-GVP requirements
✔ Scalable support for pharma, biotech, medical devices & nutraceuticals
✔ Confidential, transparent & inspection-ready processes
🛡️ Protect Patients. Protect Your Brand.
Outsource your Safety & Complaint Handling to experts who understand compliance—so you can focus on innovation and growth.
📩 Contact CMPV Consultancy LLP today
to strengthen your safety systems and stay inspection-ready.

Leave a comment