ICSR Management
Comprehensive and Reliable Pharmacovigilance Solutions
Who We Are
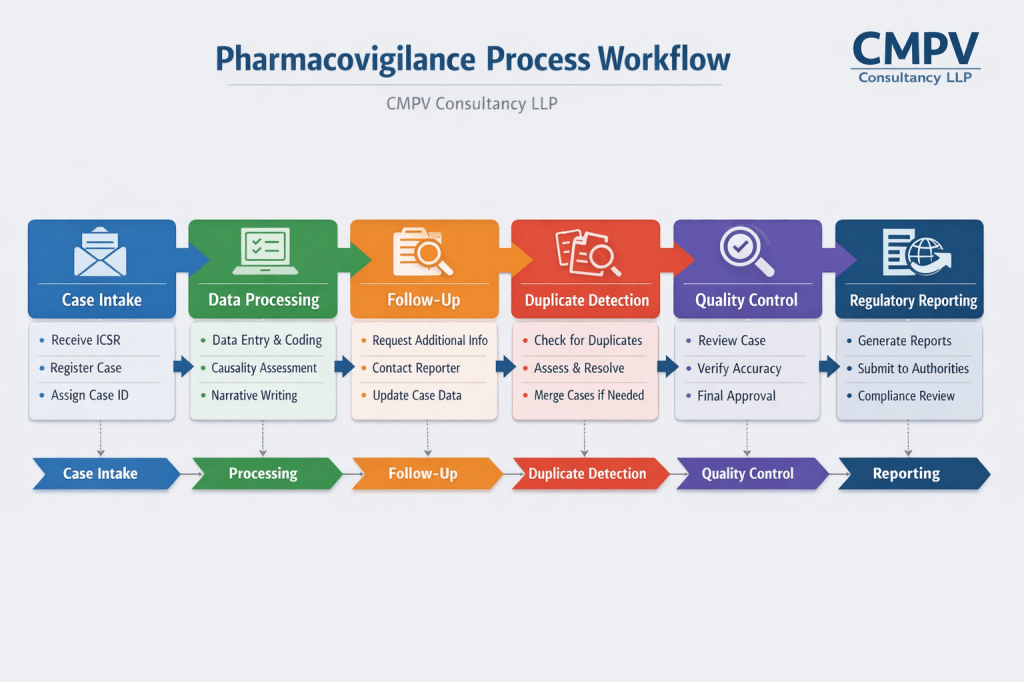
CMPV Consultancy is a premier provider of pharmacovigilance solutions, specializing in the comprehensive management of Individual Case Safety Reports (ICSRs) for the pharmaceutical industry and Contract Research Organizations (CROs). With a team of dedicated experts, we ensure accurate and timely reporting that meets global regulatory standards.
Our Services
Our ICSR management services are tailored to enhance the efficiency and reliability of your drug safety operations. Our key offerings include:
- ICSR Processing and Reporting: Efficient collection, processing, and submission of ICSRs to relevant regulatory authorities, ensuring compliance with all regulatory requirements.
- Signal Detection: Utilization of advanced analytics to identify and analyze signals from individual case reports, enhancing drug safety and risk management.
- Data Quality Management: Ensuring high standards of data integrity and accuracy in the reporting and analysis of ICSRs.
- Regulatory Submission: Automated and manual submissions to all required regulatory bodies, including EMA and FDA, in the appropriate formats.
- Training and Education: Providing comprehensive training sessions to ensure your team is well-versed in the latest ICSR management practices and regulatory changes.
- Audit Support: Assistance in preparing for and responding to audits and inspections related to ICSR processes.
Why Choose Us?
- Expertise: Our team consists of pharmacovigilance professionals with extensive experience in handling ICSRs across various therapeutic areas.
- Technology-Driven: We leverage the latest technology and software tools to streamline the management of ICSRs, ensuring fast and accurate reporting.
- Customized Solutions: We understand that each client has unique needs. Our services are highly customizable to align with your specific requirements.
- Global Compliance: Our deep understanding of international pharmacovigilance regulations ensures that your ICSR management is fully compliant with global standards.
- Proactive Communication: We believe in maintaining open lines of communication with our clients to keep you informed every step of the way.
Our Commitment
At CMPV Consultancy, we are dedicated to supporting our clients in meeting their pharmacovigilance obligations while maximizing drug safety and compliance. Our approach is centered on providing meticulous, timely, and effective ICSR management to safeguard patient health and support regulatory success.
Contact Us
Discover how our ICSR management services can transform your pharmacovigilance operations. Contact CMPV Consultancy today to schedule a consultation or to learn more about how we can assist you.
Your message has been sent
Ph. +91 9337504500
cmpvconsultancy@gmail.com
Partner with CMPV Consultancy for expert management of your pharmacovigilance data. We are here to help you navigate the complexities of drug safety reporting and ensure excellence in every aspect of your operations.
